Access procedure
Collaboration and access
GARBH-INi DRISHTI provides researchers with a comprehensive overview of available data and clear guidelines for requesting access. By providing de-identified datasets, the platform upholds participant confidentiality while fostering collaborative research to drive advancements in maternal and child health.
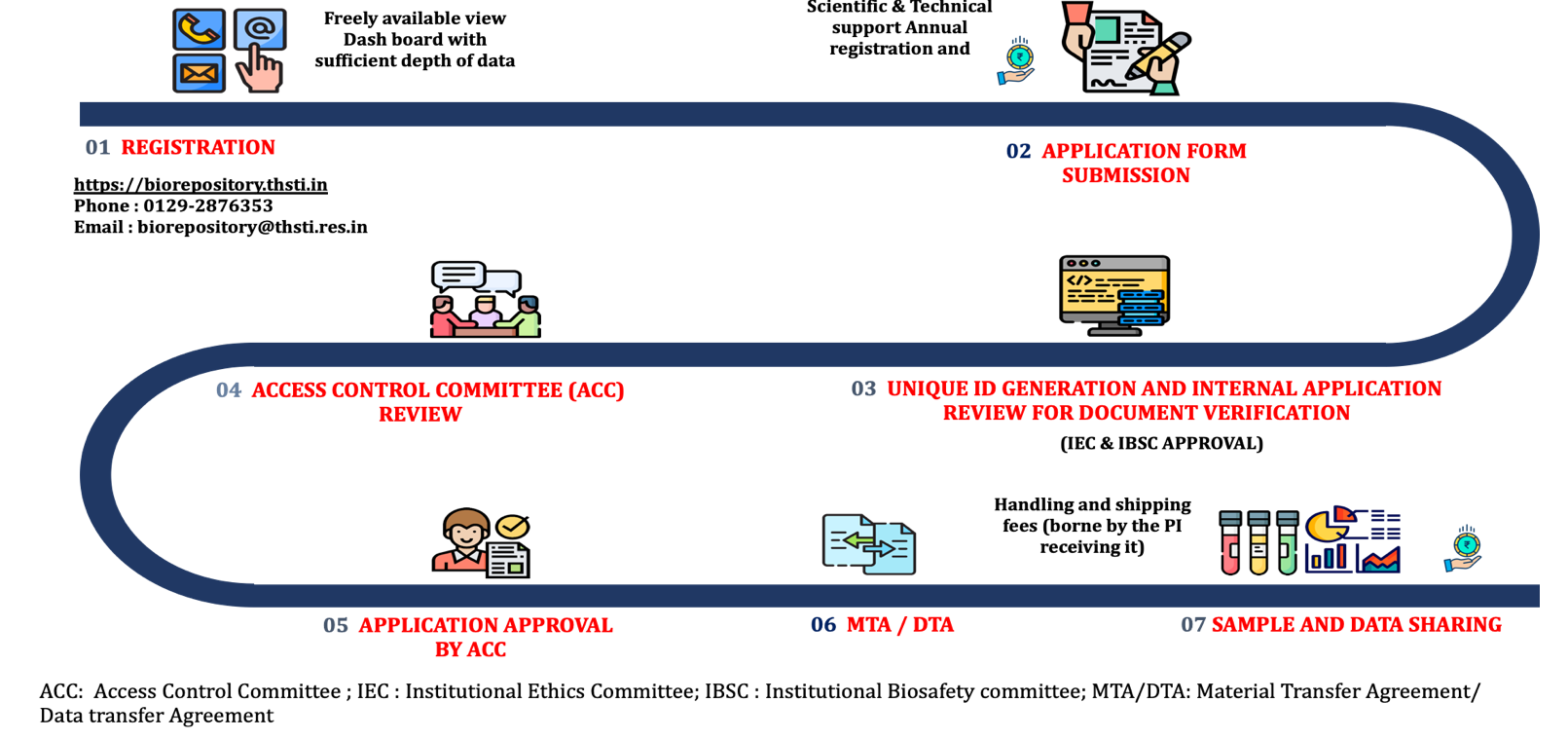
Step by step procedure to access and sharing of data and sample
The approval for all requests from external Principal Investigators, will be routed through a DBT-nominated GARBH-INi Access Control Committee (GACC), consisting of members from the funding body, research and medical institutions, including domain experts.
Step 1: REGISTRATION BY THE USER:
Provide necessary information such as name, organization name, email & contact number along with a brief CV with publication details.
Evidence that the institute is a legitimate research entity with prior experience in health-related research will be confirmed. Once verified a nominal annual registration fee will be requested and USER LOGIN will be activated.
Step 2: APPLICATION FORM SUBMISSION:
Submit the completed Data/Sample Access Request form and Letter of Intent to Member Secretary of GACC at biorepository@thsti.res.in
Step 3: UNIQUE ID GENERATION AND INTERNAL APPLICATION REVIEW FOR DOCUMENT VERIFICATION:
UID will be provided to each requester after internal review for Data/Sample availability along with document verification (IBSC/IEC/Funding details).
Step 4: GARBH-INi ACCESS CONTROL COMMITTEE (GACC) REVIEW:
Peer review of the submitted proposal will be done based on scientific merit, usefulness in terms of public health importance, feasibility, appropriate use, ethical appropriateness and novelty of the proposal.
Step 5: APPLICATION APPROVAL BY GACC:
Users will be notified about the approval status through email.
Step 6: DATA/ MATERIAL TRANSFER AGREEMENT (DTA/MTA):
User notification for DTA/ MTA will be shared to the applicant for execution of these documents.
Step 7: DATA AND SAMPLE SHARING:
Users have to make their own arrangements for sample shipment following the regulatory guidelines.
Data will be shared on the registered email ID of the users through secure servers.