Explore Data
Data Overview
GARBH-INi DRISHTI is a data dashboard that provides a comprehensive overview of one of South Asia’s largest pregnancy (GARBH-INi) cohort datasets.
GARBH-INi is designed to generate critical insights into maternal and child health. By systematically collecting comprehensive clinical, epidemiological, imaging and biospecimen data at multiple time points throughout pregnancy and the postpartum period, GARBH-INi enables pioneering research aimed at enhancing maternal and neonatal outcomes.
Eligibility criteria for participant enrolment into the GARBH-INi cohort
Screening at the time of first antenatal visit to the hospital:
Inclusion Criteria
- Willingness to visit the study site during the scheduled study time points for the entire study duration.
- Period of gestation, as per the last menstrual period (LMP), is ≤19 weeks and 6 days.
- Written informed consent to participate in the study.
Enrolment
Inclusion Criteria
- Fulfill screening criteria and has an ultrasound confirmation of an intrauterine pregnancy with gestational age <20 weeks
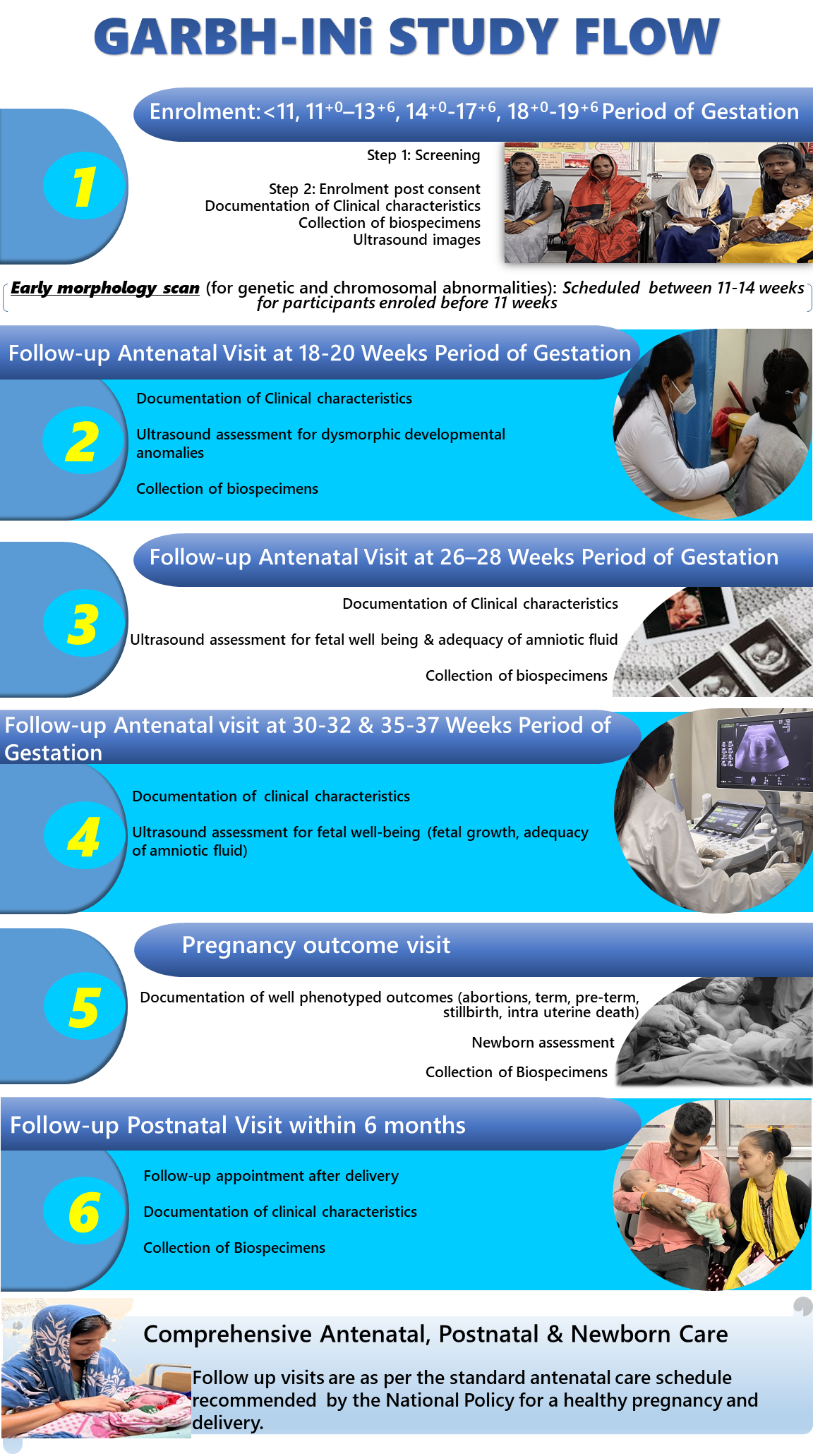
Participants enroled (≤19 weeks and 6 days gestation) in the GARBH-INi cohort undergo comprehensive assessments during pregnancy at recommended time periods (18-20, 26-28, 30-32, and 35-37 weeks of gestation) as outlined in the figure.
These assessments include the collection of clinical and epidemiological information, ultrasound imaging to evaluate fetal and maternal characteristics and collection of varied biospecimens.
Participants are followed through delivery, during which clinical and biological data is collected from the mother-baby dyad. Post-natal follow-ups continue to capture critical maternal and neonatal health outcomes.
Clinical variables
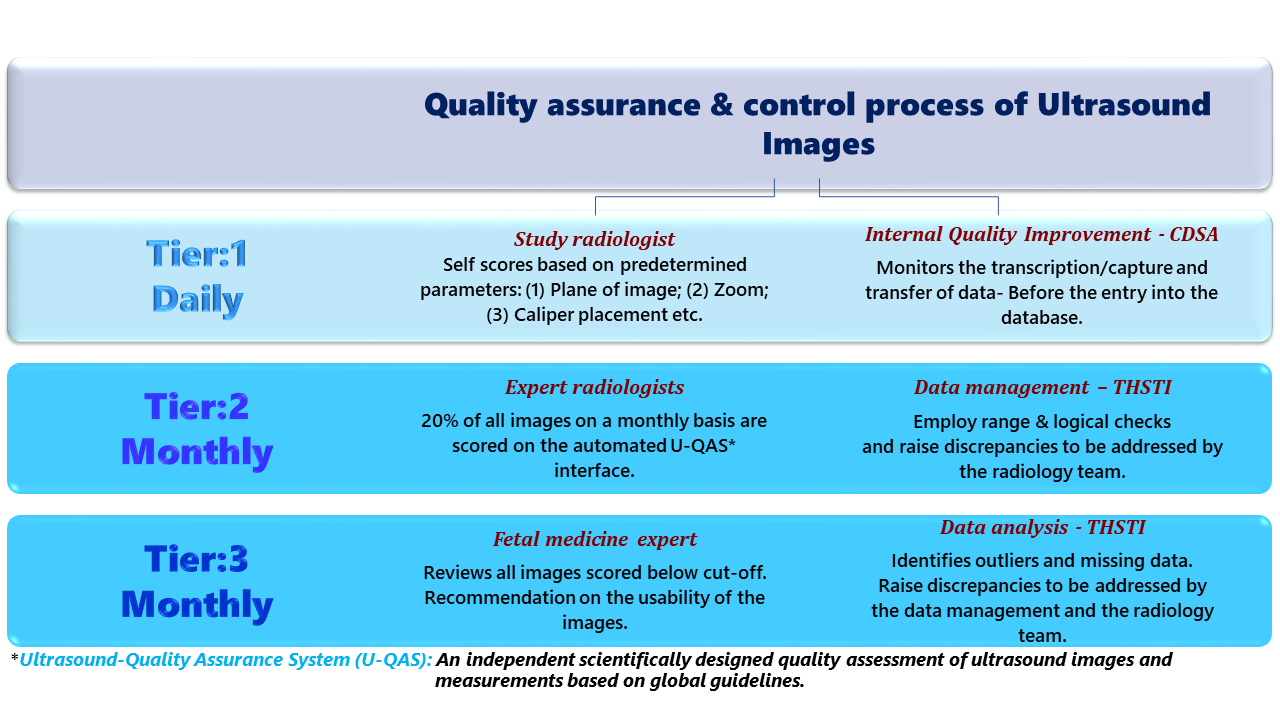
Quality Control
Comprehensive quality control and standardization measures ensure the accuracy, reliability, and consistency of the data collection.
- Regular training of the research team to minimize inter- and intra-observer variabilities.
- Concurrent monitoring for logical, measurement, and transcription errors by internal quality improvement team.
- Weekly process monitoring of maternal and neonatal anthropometry.
- Regular standardization of study equipment.
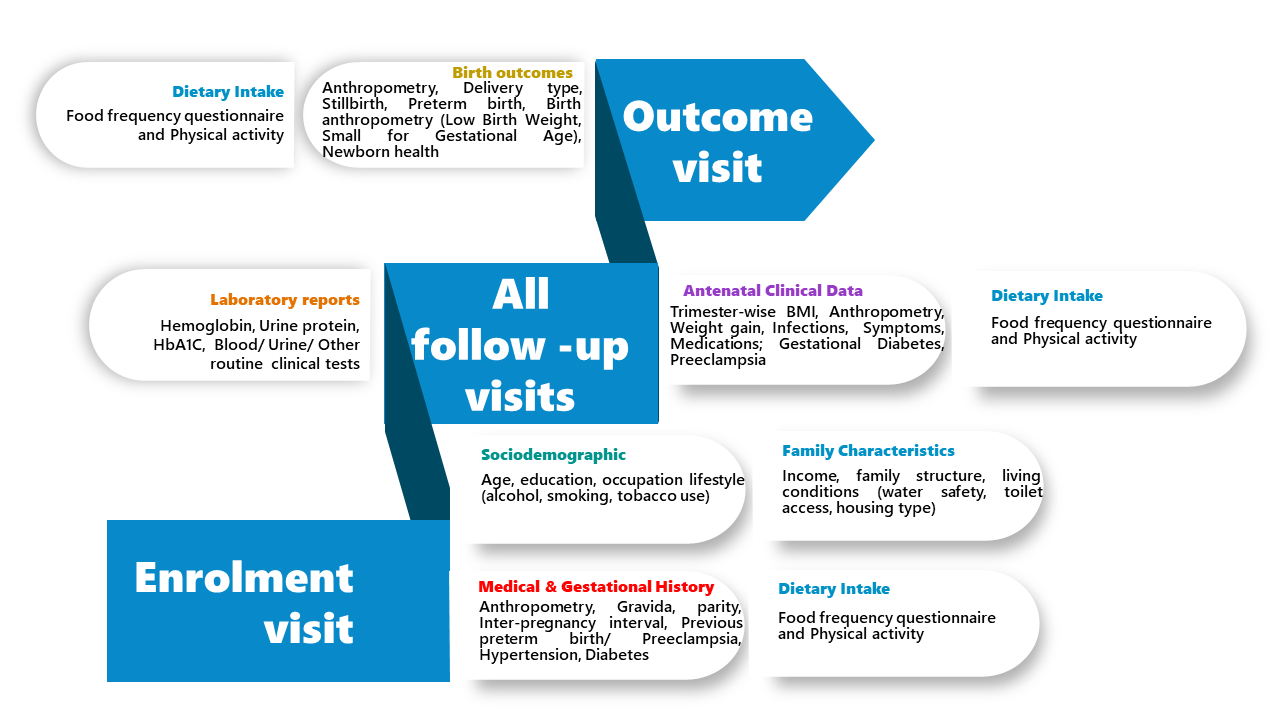
Clinical and Epidemiological Data: Detailed participant data, including demographic, clinical, and health records, are collected at each visit.
Ultrasound scan variables
Critical information on fetal well-being collected longitudinally across pregnancy.
Serial images are taken by a dedicated study radiologist on a GE Voluson E8 Expert (General Electric Healthcare, Chicago, Illinois) ultrasound machine at the study site.
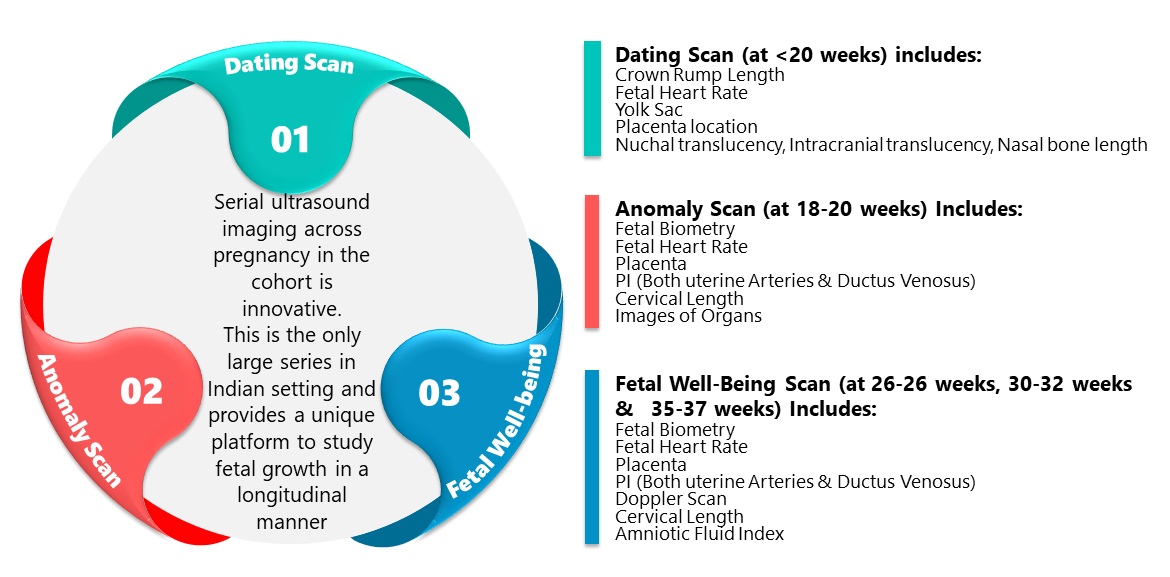
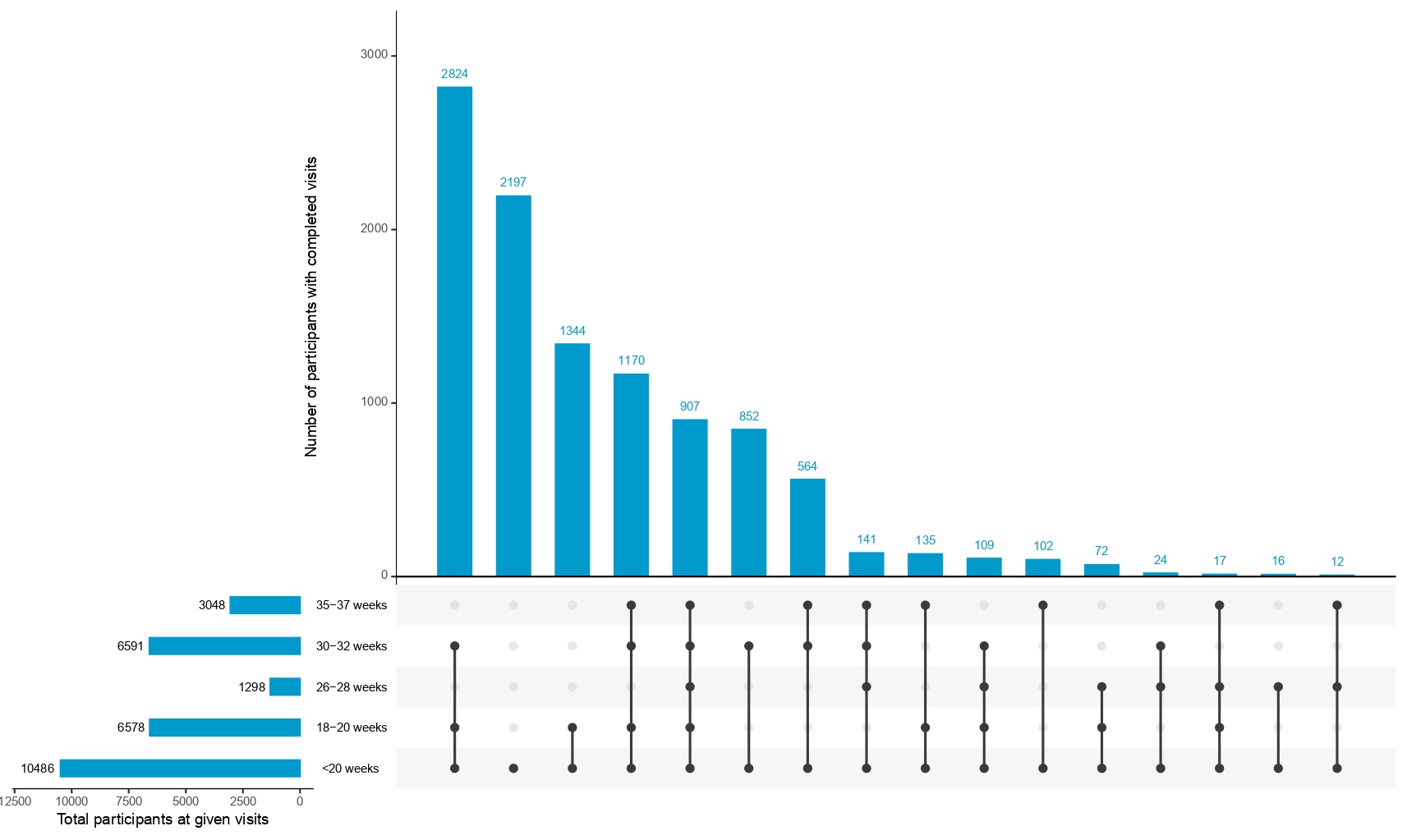
Abdominal ultrasound scans are performed to assess Period of Gestation (POG), fetal growth & well-being, placental location & morphology, as well as the morphological markers for genetic and chromosomal abnormalities at 11–14 weeks. Dysmorphic developmental anomalies are evaluated by ultrasound scans done at 18–20 weeks.
Serial changes in cervical length are monitored using transvaginal scans.
Doppler scans scheduled between 26-28 weeks, 30 and 32 weeks and 35-37 weeks POG evaluates uterine and umbilical artery pulsatility indices and amniotic fluid index as markers of fetal wellbeing; fetal middle cerebral artery and ductus venosus blood flow are measured as potential markers of fetal growth restriction.
Quality control is ensured through standardized protocols, monthly review of images by experienced radiologists and training and retraining of the study radiologists.
Coordination for submission of de-identified ultrasound imaging data with IBDC ongoing
High quality ultrasound data for preterm risk prediction model, measuring fetal biometry and assessment of other feto maternal characteristics
Biospecimens
- First DBT supported biobank accredited with ISO 20387: 2018 standards, adhering to internationally recognised biobanking best practices. Member of the International Society for Biological & Environmental Repositories (ISBER). Participation in global proficiency testing programs.
- Hosting over 1.4 million biospecimens from the GARBH-Ini cohort.
- Efficient management of sample tracking, data handling, and retrieval utilizing a sophisticated Laboratory Information Management System (LIMS)
- Regular quality checks and audits for very high standards of preservation and data accuracy.
- Real-time dashboards and automated workflows for efficient data management.
- Pre-pandemic sera/plasma panels as control samples during COVID-19 Pandemic research activities.
- Specialized training initiatives to empower the biobanking community.
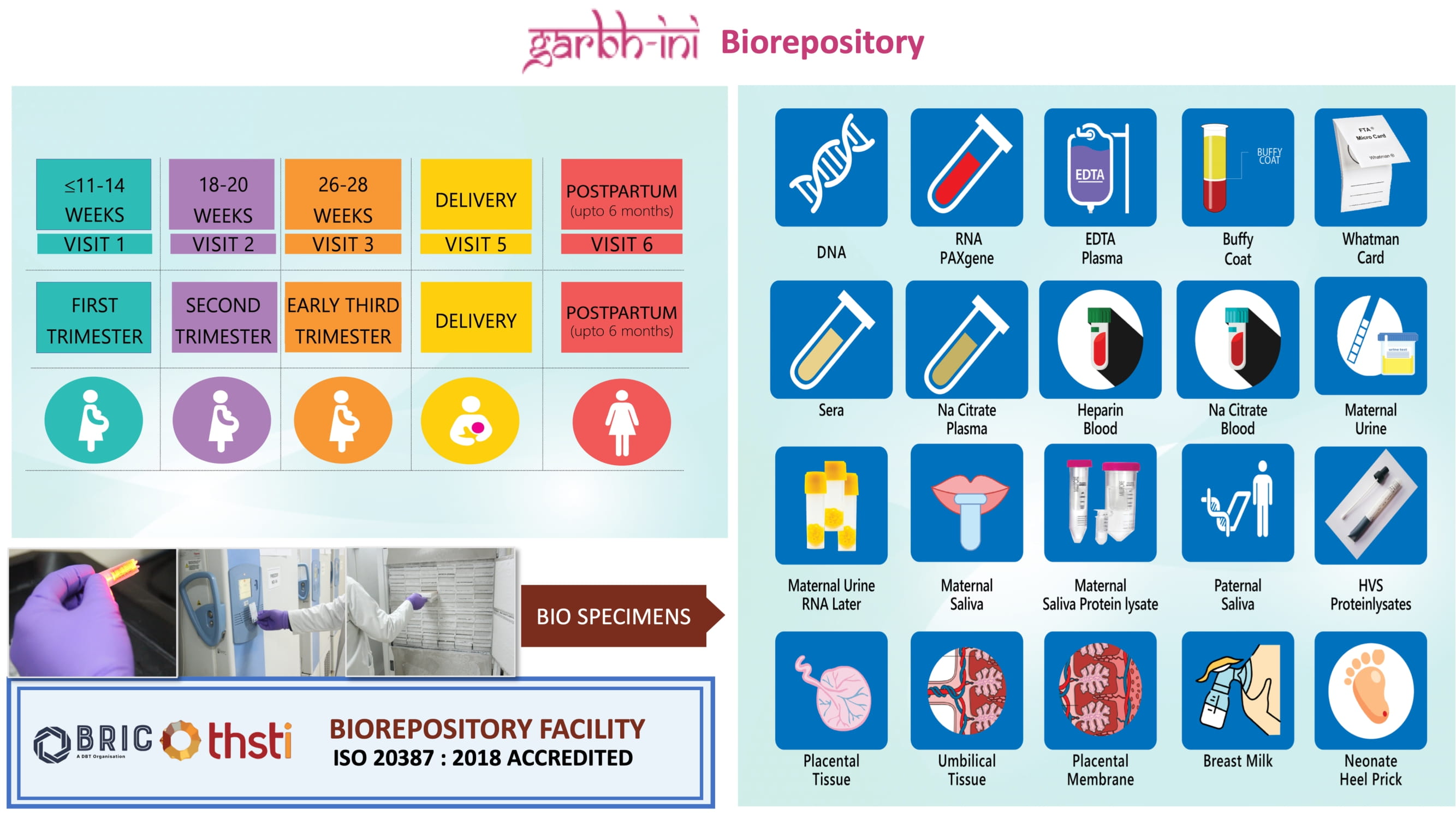
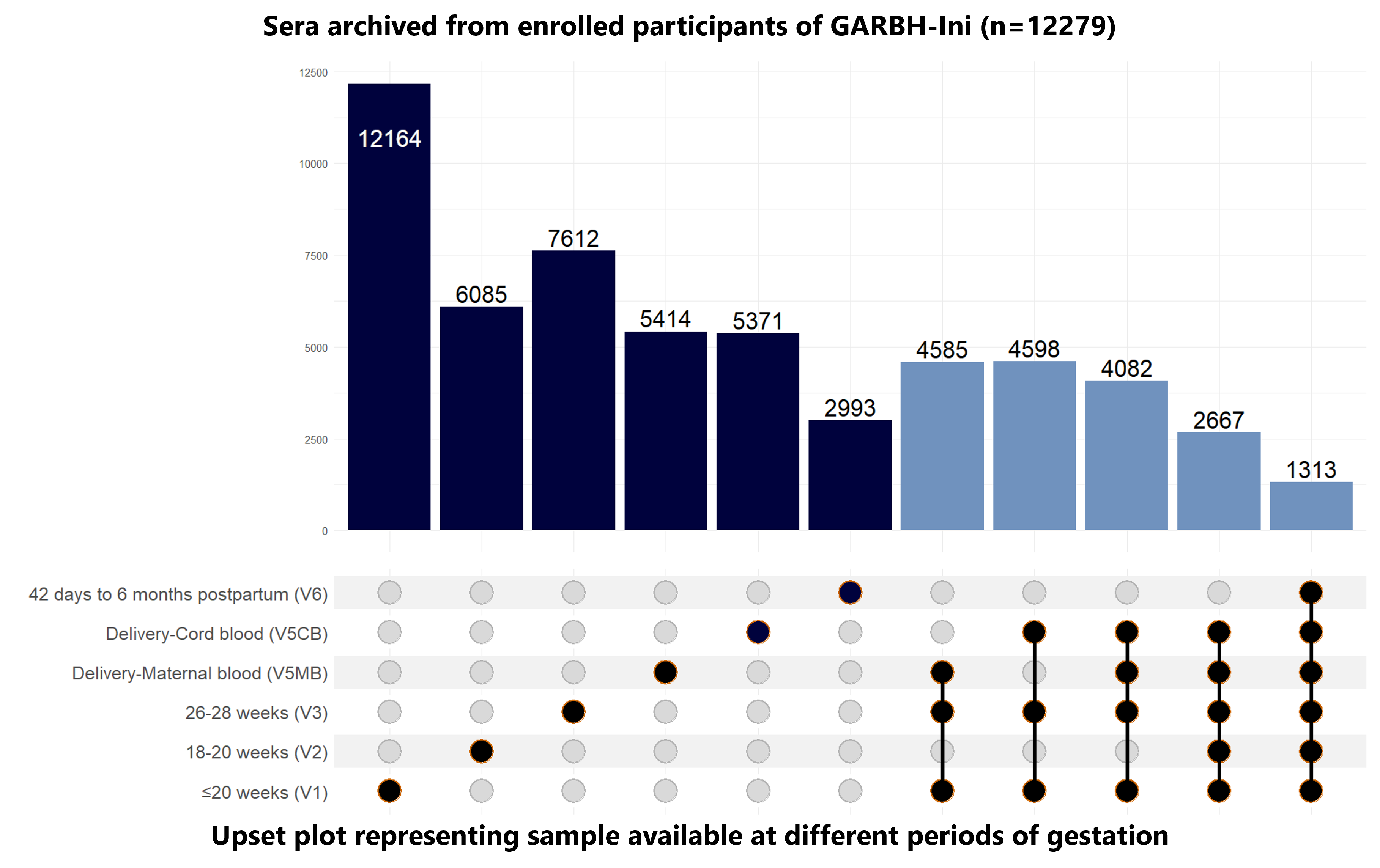
Click here for more information on biospecimens collected at different periods of gestation
First DBT-supported biobank accredited with international standard ISO 20387:2018 'Biotechnology–Biobanking- General Requirements for biobanking
OMICS Data
Coordination for submission of genomics and proteomics data with IBDC is ongoing
GARBH-INi was initiated with the hypothesis that preterm birth (PTB) may arise from a complex interplay between static genomic predispositions and dynamic modulations, including epigenomic, proteomic, metabolomic, and microbiomic factors, at various stages throughout pregnancy. Therefore, multidimensional data acquired on high-throughput platforms in a longitudinal manner across pregnancy has been analysed with the aim of stratifying women into defined risk groups for PTB. Advancements in microbiome research have facilitated the development of dipstick-based assays for the rapid detection of microbes associated with preterm birth. Additionally, Lactobacilli consortia have emerged as promising nutraceuticals, aimed at improving maternal health and reducing the risk of infections during pregnancy. Genome wide analysis of GARBH-INi women has identified maternal SNPs associated with sPTB, which are being taken forward as leads for development of SNP panels. Proteo-metabolic signatures are being validated for development of biomarker panels to predict PTB. This multi omics data is being analysed in parallel to identify physiological and molecular pathway mechanisms that are altered in relation to PTB that may have causal links and can lead to possible discovery of candidate signatures/leads.
Link to GWAS catalogue login page given below
- https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE216906 for the study accession number GSE216906
- https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE169338 for the study accession number GSE169338
- https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE169338 for the study accession number GSE234190
httpss://ibdc.dbtindia.gov.in/ipd/browse-data.php
GARBH-INi Multiomics Approach: Embracing Healthier Beginnings
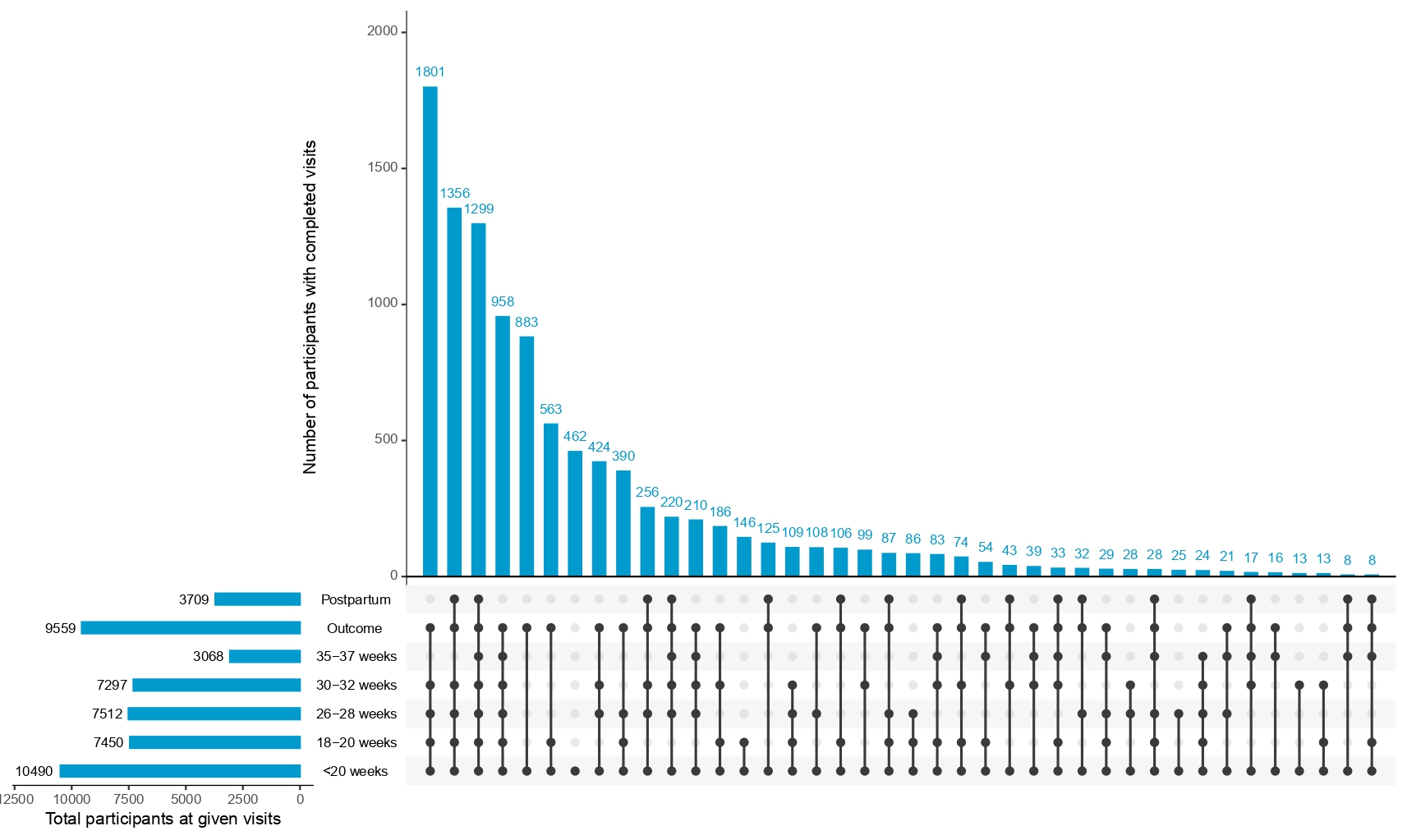
How to Interpret Upset Plots
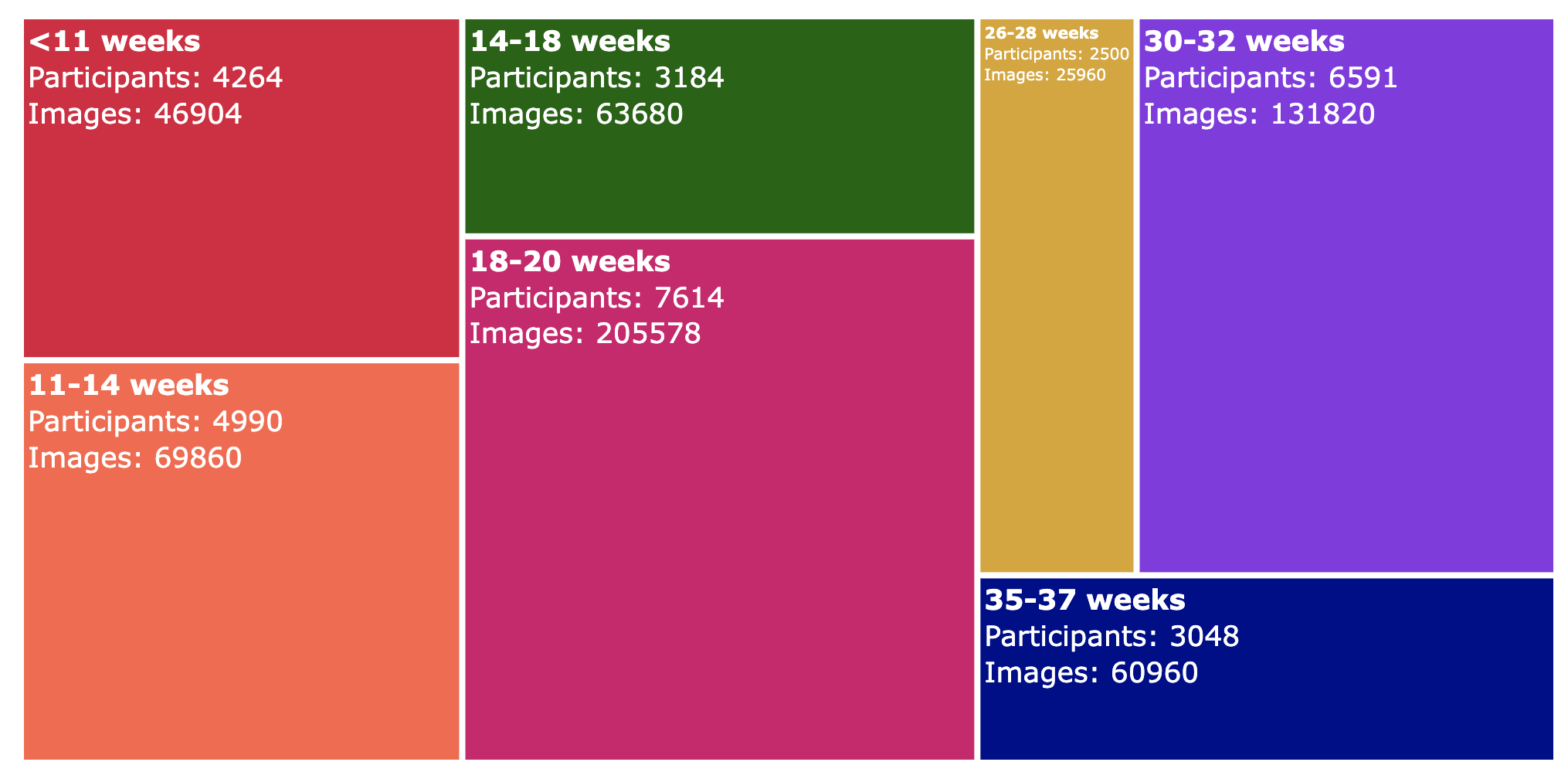
How to Interpret This Treemap
- Each block represents a time in pregnancy – The labels (e.g., "<11 weeks", "11-14 weeks") indicate the gestational age range.
- Block size reflects data volume – Larger blocks mean more data was collected in that time period.
- Colour differentiates categories – Each gestational age group has a distinct colour for easy identification.
- Numbers indicate data points – The numbers inside each block represent the data collected for that gestational period.
- Compare easily – You can quickly see which periods have the most and least data contributions based on block size.
